Edition VMT011
- Directors update: Dr Donald Sibanda and Dr Sam Beckett
- New product registrations
- Useful information for industry
- Assessment, Investigations and Monitoring – Importation of unregistered veterinary chemicals
- List of new, suspended or cancelled manufacturer licences
- Applying for Item 24 and 24V applications for pharmacopoeia standard actives
Directors update: Dr Donald Sibanda and Dr Sam Beckett
Welcome to the July 2023 edition of the Australian Pesticides and Veterinary Medicines Authority’s (APVMA) Veterinary Medicines Regulatory Newsletter. In this edition we have included information on several regulatory topics, including a new section 6A Guideline, information on applying for Item 24 and Item 24V applications for pharmacopoeia standard actives, and monitoring the importation of unregistered veterinary chemicals.
We are also pleased to announce that Mr Rodney Edmundson has been permanently appointed to the position of Director, Permits and Minor Use. Mr Edmundson is looking forward to continuing to work with stakeholders seeking authorisation of veterinary medicines under permit.
We continue to encourage applicants to participate in the APVMA stakeholder meetings, which are conducted from February to October. These meetings are a valuable forum for applicants, industry, and the APVMA Veterinary Medicines Team to discuss current and future applications and priorities and to work through any concerns. These meetings can be organised through your case manager. If at any time you require assistance with matters related to the registration of veterinary medicines, please reach out to the Veterinary Medicines Team.
If you have any suggestions or requests for content to be included in future editions of the APVMA’s Veterinary Medicines Regulatory Newsletter, please send them to the APVMA Communications Team.
New product registrations
The APVMA publishes a fortnightly Gazette, which includes information about new product registrations and variations to currently registered products, particulars, or conditions of registration.
New veterinary product registrations based on a new active constituent published in the Gazette during the April to June 2023 quarter are listed in Table 1.
|
Application no. |
Product name |
Active constituent/s |
Applicant name |
Description of the application and its purpose |
Date of registration |
Product registration no. |
|---|---|---|---|---|---|---|
|
130243 |
Nexgard Spectra Spot-On for Cats 2.5 – 7.5 kg |
83 g/L praziquantel, 12 g/L esafoxolaner, 4 g/L eprinomectin |
Boehringer Ingelheim Animal Health Australia Pty Ltd |
Registration of 83 g/L praziquantel, 12 g/L esafoxolaner, 4 g/L eprinomectin topical spot-on solution product for the treatment and control of internal and external parasites in cats, in conjunction with approval of active constituent esafoxolaner for use in veterinary chemical products. |
27 March 2023 |
90860 |
|
130244 |
Nexgard Spectra Spot-On for Cats 0.8 – 2.4 kg |
83 g/L praziquantel, 12 g/L esafoxolaner, 4 g/L eprinomectin |
Boehringer Ingelheim Animal Health Australia Pty Ltd |
Registration of 83 g/L praziquantel, 12 g/L esafoxolaner, 4 g/L eprinomectin topical spot-on solution product for the treatment and control of internal and external parasites in cats, in conjunction with approval of active constituent esafoxolaner for use in veterinary chemical products. |
3 April 2023 |
90861 |
|
128769 |
Vaxsafe MG304 |
Each dose contains >105.7 CCU living, attenuated, temperature-sensitive Mycoplasma gallisepticum vaccine strain ts-304. |
Bioproperties Pty Ltd |
Registration of a lyophilised vaccine containing live Mycoplasma gallisepticum (MG) strain ts-304 (≥105.7 CCU/dose) for the aid in the control of chronic respiratory disease caused by Mycoplasma gallisepticum in susceptible (MG free) chickens at risk of infection. |
31 March 2023 |
90403 |
|
133781 |
Bravecto Quantum Fluralaner 150 mg/mL Injectable Suspension for Dogs |
150 mg/mL fluralaner |
Intervet Australia Pty Ltd |
Registration of a 150 mg/mL fluralaner injection product for the treatment and control of flea infestations, control of brown dog and paralysis ticks and control of flea allergy dermatitis in dogs |
28 April 2023 |
91883 |
|
132669 |
Flexolt Oral Lice Treatment for Sheep with any Length of Wool |
10 g/L fluralaner |
Intervet Australia Pty Ltd |
Registration of 10 g/L fluralaner lousicide oral solution for sheep, for the control of isoxazoline-susceptible lice (Bovicola ovis), including strains resistant to synthetic pyrethroids and insect growth regulators, on sheep and lambs with any length of wool |
31 March 2023 |
91565 |
Useful information for industry
In this edition we’ve included an update on our new section 6A Guideline, recent website updates, and finalisation modules for combined Item 10, 24 and 24V applications.
New 6A Guideline: Requesting information from applicants
The APVMA is currently reviewing our suite of section 6A Guidelines, to ensure they are relevant to the decision-making process of APVMA staff. As noted in our March 2023 newsletter, the guidelines are being updated progressively to refine the information and ensure the content is suitable for a 6A Guideline compared with general information and guidance for applicants.
On 8 June 2023, the APVMA’s Interim Deputy Chief Executive Officer approved publication of a new 6A Guideline ‘Requesting information from applicants’. This guideline includes guidance for staff on the 3 main provisions in the Agvet Code used to request information from applicants during the assessment of an application:
- Preliminary assessment notice of defects (ss 11(3), 28(3), 110A(3))
- Updating or clarifying information (under section 8C(a)(iii) and regulation 8AHAA taken together)
- Notice of requirement of additional information, report or sample (s 159)
The guideline is presented in a format that provides the following content:
- The Agricultural and Veterinary Chemicals Code Act 1994 (Agvet Code) provision to which the guidance relates.
- An explanation of the provision as deemed suitable.
- The matters APVMA staff need to take into account when considering the relevant decision.
- Legislative references and examples to provide additional context and information.
The previous 6A Guideline, ‘Section 159 in the context of applications under the Agvet Code’ has been revoked; however, is retained on our website for information purposes.
Under section 6A of the Agvet Code, the APVMA may make written guidelines for performing its functions and exercising its powers under the Agvet Code. If the APVMA makes a 6A Guideline, staff must have regard to it when making decisions.
Finalisation modules for combined Item 10 applications and Item 24/24V applications
Combined Item 10 applications finalisation modules
Combined Item 10 applications are to both register a new chemical product and approve new source(s) of active constituent(s). This includes registration of a new chemical product that is ‘closely similar’ to a reference product and approval of a new source of active constituent.
Module 1.0 will apply to all Item 10 applications. The assessment modules assigned to these applications will be the appropriate modules for the product registration, plus an additional module 2.5 for each new source of pharmacopoeia active constituent and/or an additional module 2.3 for each new source of non-pharmacopoeia active constituent.
The finalisation module assigned to the ‘combined’ Item 10 application will either be a module 11.1 or module 11.2.
- Module 11.1 will apply if there are 3 or more technical assessment modules (module 2.3 and above) on the application; or if the assessment requires consideration of 3 or more assessment reports from technical assessment modules conducted under previous applications.
- Module 11.2 will apply in all other cases.
Item 24/24V applications finalisation modules
As outlined in the APVMA’s current module descriptors, module 11.3 (finalisation level 3) is applied to applications for 'approval or variation of an existing active constituent'.
For pharmacopoeia standard actives, Item 24/24V applications will usually have modules 1.0 (preliminary assessment), module 2.4 or module 2.5 and module 11.3. Module 12.0 may be applied if the information submitted with the application requires data protection.
Retirement of ‘fast-track’ option for Item 8 applications
On 1 July 2023, the APVMA retired the 21-day service level standard ‘fast-track’ option for Item 8 applications, which are applications to register a product that is the same as a reference product (also known as a ‘repack’).
The fast-track option was introduced in 2016 for Item 8 applications that met certain criteria. The APVMA’s service level standard has been to complete assessment of these applications in 21 days instead of the 3-month statutory timeframe. However, recent changes to the legislation made it unfeasible for the APVMA to continue to meet this service level standard.
Item 8 applications received up to 30 June 2023 will be processed in accordance with the 21-day service level standard, provided no additional or clarifying information is required. Item 8 applications received from 1 July 2023 onwards will be processed within the statutory 3-month timeframe.
Recent website updates, including the efficacy guideline
One-stop shop: WAAVP Guideline for evaluating the efficacy of parasiticides against ectoparasites of ruminants.
As many of our stakeholders will be aware, the WAAVP guidelines for evaluating efficacy – which the APVMA has adopted for the assessment of ectoparasiticides in ruminants – have been consolidated into a single updated document.
Following this update, we have now structured the corresponding information on our website to align with the WAAVP Guideline. These changes are structural only and do not have any impact on registration requirements at this time.
We intend to undertake a technical review of this content in the near future and will notify stakeholders when this begins.
Other website updates
In July 2023, we updated the ‘Scientific references or extrapolated scientific argument’ section of the Efficacy and target animal safety general guideline (Part 8). Additional guidance has been provided on how applicants can make better use of scientific papers in their efficacy and safety dossiers to support registration/variation applications.
We have also updated the Guidelines for testing of intramammary preparations for treatment of bovine mastitis to remove repetitive text and the guidelines for labels of intramammary products, as this information can now be found in the Veterinary Labelling Code. No changes have been made to technical requirements, but we intend to undertake a technical review of this content in the near future.
Other important APVMA updates
- An update from the APVMA Board meeting held on 31 May 2023 was published on our website in June.
- Consultation on our review of guidelines for determining a minor use closed on 15 June 2023. The APVMA is now considering the submissions received.
- The Veterinary Medicines Team is currently holding a public consultation for Mometamax Ultra Ear Drops Suspension for Dogs containing Posaconazole, which closes on 25 July 2023.
- Don’t forget to subscribe to receive notifications when the APVMA publishes recalls on our website.
Assessment, Investigations and Monitoring – importation of unregistered veterinary chemicals
The APVMA’s Assessment, Investigations and Monitoring (AIM) Team is responsible for monitoring compliance with the agvet legislation and conducting investigations where suspected breaches are detected. The AIM Team works collaboratively with other government agencies to detect non-compliance activity, including the Australian Border Force, in detecting unlawful importations of unregistered veterinary chemicals.
Without the approval of the APVMA, a veterinary chemical cannot be legally imported, marketed, supplied, or used in Australia. Section 69B of the Agricultural and Veterinary Chemicals (Administration) Act 1992 makes it an offence for a person to import an unapproved active constituent or unregistered chemical product into Australia. In instances where an unapproved active constituent or unregistered chemical product is imported unlawfully into Australia, the APVMA will request the Australian Border Force to seize the goods as a prohibited import and have those goods forfeited to the Crown in accordance with section 229 of the Customs Act 1901.
The APVMA recognises the value of information reported by industry and the community to detect, deter, disrupt, and report non-compliance in the veterinary chemical sector. If you are aware of any information relating to the actual or suspected importation of an unregistered veterinary chemical, you are encouraged to make a report through our online non-compliance reporting form. If you wish to discuss a non-compliance matter, learn how to report a matter, or are unable to submit an online non-compliance report, please contact compliance@apvma.gov.au.
More information about the investigations conducted by and the enforcement outcomes of the AIM Team can be found on our website.
New, suspended or cancelled manufacturer licences
The APVMA’s Manufacturing Quality and Licensing (MQL) Team gazettes a list of licences for new manufacturers issued under subsection 123(1) of the Agvet Code and licences suspended or cancelled under subsection 127(1) the Agvet Code.
Tables 2 and 3 list the new licences and licences suspended or cancelled since 16 February 2023.
You can view the complete list of currently licenced Australian manufacturers on our website.
|
Company name |
Licence number |
Company ACN |
Address |
Product types |
Steps of manufacture |
Date issued |
|---|---|---|---|---|---|---|
|
Virbac (Australia Pty Ltd) Pty Ltd |
6137 |
003 268 871 |
361 Horsley Rd |
Category 6: (single step manufacture) – all dosage forms |
Quality assurance (QA) of packaging materials, storage and release for supply |
16 February 2023 |
|
DHL Supply Chain (Australia) Pty Ltd |
6071 |
071 798 617 |
16 Picrite Cl |
Category 6: all product dosage forms |
Secondary packaging, secondary/supplementary labelling, storage, and release for supply. |
1 March 2023 |
|
AsureQuality Australia Pty Ltd |
1085 |
106 787 704 |
28 Mareno Rd |
Category 1: Immunobiologicals |
Quality assurance (QA) of raw materials, aseptic filling, secondary packaging, labelling, storage and release for supply. |
21 March 2023 |
|
Australian Life Sciences Pharma Pty Ltd |
2250 |
604 520 532 |
Unit 2–3 |
Category 2: Pastes, powders, creams and ointments |
Quality assurance (QA) of raw materials, formulation including blending, filling, packaging, labelling, analysis and testing (chemical, physical), storage, and release for supply. |
22 March 2023 |
|
Equivet Pty Ltd |
2034 |
003 837 492 |
37 Lord Fury Ct |
Category 2: pastes and powders |
Quality assurance (QA) of raw materials, formulation including blending, filling, packaging, labelling, analysis and testing (physical), storage, and release for supply. |
5 April 2023 |
|
Hall Family Group Pty Ltd |
6198 |
166 410 671 |
Unit 3, 30 Heathcote Rd |
Category 6: (single-step manufacture) – all dosage groups |
Filling, packaging, labelling and storage. |
12 April 2023 |
|
Lavida Nutraceuticals Pty Ltd |
2278 |
168 386 472 |
1/25 Wonderland Dr |
Category 2: tablets, capsules – hard shell, creams, lotions, ointments, liquids (oral and topical), powders, granules, sprays |
Quality assurance (QA) of raw materials, formulation including blending, dry milling, wet milling, granulation, filling, packaging, labelling, blister packaging, sachet packaging, tableting, tablet coating, capsule filling from bulk, analysis and testing (physical, chemical and microbiological), storage and release for supply. |
17 April 2023 |
|
AB Initio Pharma Pty Ltd |
2277 |
633 008 134 |
Level 1, 67–73 Missenden Rd |
Category 2: Spray, aerosols, liquids, suspensions, cream, gels, ointments, paste; capsule (hard shell), powder and granules |
Quality assurance (QA) of raw materials, formulation including blending, filling, capsule filling from bulk, aerosol filling from bulk, freeze-drying, packaging, labelling, analysis and testing (physical and chemical), storage and release for supply. |
26 April 2023 |
|
Treidlia Biovet Pty Ltd |
1096 |
150 496 138 |
Unit 76, Powers Business Park |
Category 1: Immunobiologicals |
Quality assurance (QA) of raw materials, bacterial fermentation, fungal fermentation, wart tissue extraction, pilot scale affinity chromatography, formulation including blending, filling, aseptic filling, packaging, labelling, sterilisation (heat, chemical and filtration), microbiological reduction treatment (heat, filtration, and chemical), analysis and testing (physical, chemical, microbiological, serological, sterility testing, vaccine safety testing, and protein biochemistry) and storage. |
3 May 2023 |
|
Dermcare-Vet Pty Ltd |
1109 |
010 280 010 |
7 Centenary Rd |
Category 1: Sterile and immunobiological products |
Quality assurance (QA) of raw materials, formulation including blending, filling, aseptic filling, packaging, repackaging, labelling, relabelling, analysis and testing (physical, chemical and microbiological), storage and release for supply. |
10 May 2023 |
|
AUSVETLAB Pty Ltd |
1063 |
001 611 516 |
105 Norman Jones Ln |
Category 1: Tick antiserum and snake antivenoms |
Quality assurance (QA) of raw materials, serum collection, management and immunisation of donor animals, formulation including blending, aseptic filling, sterilisation (heat and filtration), microbiological reduction treatment (heat, filtration and chemical), packaging and labelling, analysis and testing (physical), storage, and release for supply. |
25 May 2023 |
|
Baron Rubber Pty Ltd |
6222 |
005 178 549 |
11 Northcorp Blvd |
Category 6: Rubber inserts |
Quality assurance (QA) of raw materials, formulation including blending (rubber compounding), rubber insert moulding, analysis and testing (physical), storage, packaging, labelling, and release from manufacture only (partial release). |
29 May 2023 |
|
Company name |
Licence number |
Company ACN |
Address |
Period of suspension |
|---|---|---|---|---|
|
The Commonwealth of Australia acting through the Commonwealth Scientific & Industrial Research Organisation |
1110 |
687 119 230 |
CSIRO Tissue Culture Facility |
From 27 June 2023 to 26 June 2024 |
Any questions about these licenses can be directed to the MQL Team.
Applying for Item 24 and 24V applications for pharmacopoeia standard actives
Following recent legislative changes that took effect on 1 February 2023, Item 24 applications have become a cost-effective option for applicants seeking to approve a new source or vary an existing pharmacopoeia source of active constituent.
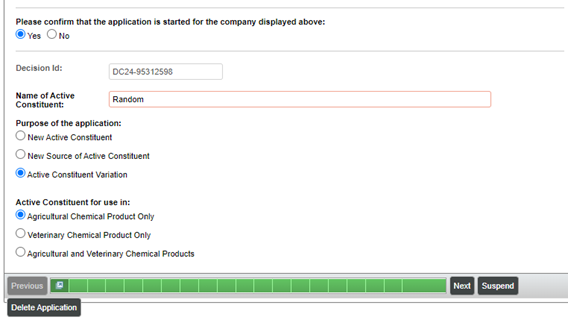
To start the Item 24 application process, you will need to obtain an Item 24 decision ID from the decision tree:
Item 24 – new source
- Select Apply for approval of a new active constituent
- Select product type – veterinary or agricultural
- Select Submit data for a reduced assessment of the active constituent where I can select the relevant modules or submit only chemistry data for assessment of an active constituent complying with an APVMA recognised pharmacopoeia – Item 24
- Select an appropriate option about consent to use information on a reference product
- Obtain an Item 24 decision ID number
Item 24 – variation
- Select Apply to vary an existing product registration, label approval or active constituent approval
- Select Active approval for This variation will apply to
- Select one of 4 options about the conditions or relevant particulars you wish to vary
- Select Complies with an APVMA-recognised pharmacopoeia monograph (BP, Ph Eur, USP) OR A non-pharmacopeia active where there is no change in the current specifications/DoC or the manufacturing process – Item 24
- Indicate if you are nominating a reference product to support your requested change
- Obtain an Item 24 decision ID number
In both cases, you will obtain an Item 24 decision ID (e.g. DC24-XXXXXXXX), and when you start your application, you will be asked to select different options. An example is shown below:
For variation applications, please remember to mention the active approval number for the active being varied in the text in the Executive Summary. Once the application is submitted to our Online Services Portal, APVMA Administration Officers will convert the Item 24 application to an Item 24V (Variation) application for processing. You will then receive an email giving notice of receipt of application, which will indicate an Item 24V application.