Table of contents
- General information
- Important – information that is published
- What constitutes information for the purposes of the information list?
- Managing sensitive information
- The information list should accurately reflect the information submitted
- Completing the information list
- Using the information list tool in the online application form
- Using the information list editor
- Overseas data submissions
- How can I get assistance?
1. General information
This document provides guidance on how to compile an information list (previously known as a data list). It provides clarity about the Australian Pesticides and Veterinary Medicines Authority's (APVMA) expectations as to what should be entered into an information list.
It is an application requirement that applications for approval, registration, or variation include a short description of each item of information contained in, or accompanying the application – an information list. This requirement does not apply to applications for registration of a chemical product that is the same as another registered chemical product where the product is to be registered with a different name (Item 8 or Item 10A), or the registration of a listed chemical product (Item 9).
It is the applicant's responsibility to ensure the information uploaded to an information list is accurate. Application lists should not contain confidential commercial information (CCI) as the information list may be published on the APVMA website as part of the registration process. It is an offence under the Agvet Code to provide false or misleading information to the APVMA.
The online application form contains a data upload tool where applicants can upload information required for applications, and enter descriptions of the information required for an information list at the same time. Alternatively, applicants who intend to provide information supporting their application by electronic media (USB) by mail may choose to develop the information list using the ‘Information List Editor (Windows Program)’ and submit the ‘.xml’ file generated by the Information List Editor with the supporting information.
2. Important – information that is published
The information list may be published on the APVMA website in application summaries, decision summaries, and on the PubCRIS database.
2.1. Application summary
When an application for approval of an active, registration of a chemical product, or variation of an approval or registration passes preliminary assessment, the APVMA is required to publish certain information – it does this in an application summary.
The application summary will include a short description of each item of information contained in or accompanying an application, including the application information details for the item of information. This is known as an information list and includes:
- the title shown on the item of information
- the name of the author, or each of the authors, of the information
- the date shown on the item of information (if any)
- if no date is shown on the item of information – the date when the preparation of the information was completed
- if the information was published, the:
- date when it was published
- name of the publication in which it was published
- a unique identifier for the item of information that indicates the location of the item in the application
- the name and address of the authorising party for the information.
The only exceptions to this obligation to publish are applications for the registration of chemical products that meet the definition of the same (Item 8 repacks or Item 10A additional label), or for the registration of a listed product (Item 9). These applications have no requirement to publish a description of each item of information contained in an application.
2.2. Decision summary
If the APVMA decides to approve or vary an active constituent, to register or vary a chemical product, or to approve or vary a label for a chemical product, it must also publish an information list showing the information relied on by the APVMA to make its decision as part of a decision summary notice. This may include a list of all or some of the information submitted with the application (and published in the application summary) in addition to any other information relied on by the APVMA to make its decision. This requirement exists for all applications granted by the APVMA (including for chemical products that are the same as a registered chemical product known as repacks).
2.3. PubCRIS
The information list is integral to the APVMA’s administration of the limits on use of information provisions of the Agvet Code. Where an approval or registration was dependent on information that is subject to a limitation period, a list of that information is published with the PubCRIS record for the product. This identifies the approval or registration as having ‘protected’ information, and allows other parties to identify authorising parties for the information from whom consent would be required, should the use of that information be sought.
It is important to be aware that if an application relies on a reference active constituent or a reference product1 that has ‘protected’ information (with the consent of the authorising party for the information), a record of any ‘protected’ information which is relied on to grant the new application will be inherited by the new active constituent or product, and this information will also be displayed in the PubCRIS record.
3. What constitutes information for the purposes of the information list?
Any document (information) submitted with the application constitutes information for the purposes of the information list. This includes supporting information such as consent for use letters, MSDS, certificates of analysis, GMP certificates, and other such documentation in addition to scientific studies or data that may be submitted to specifically address the safety, trade and efficacy criteria.
4. Managing sensitive information
4.1. Study titles
Applicants and other parties who conduct or commission studies for the purpose of supporting applications made to the APVMA should be mindful that the information list containing certain details about each item of information will be published, and name each study appropriately.
The APVMA appreciates that, for certain types of information, the applicant is unable to influence the title shown on the item of information. This is particularly the case for GMP certificates, MSDS, certificates of analysis, or consent for use letters where the title or author of the document might reveal the manufacturer or constituents of the product. For this reason, it is acceptable to the APVMA for applicants to consolidate the following types of information into a single document within the information package submitted with an application, with a single entry in the information list:
- GMP certificates for manufacturers of the product (veterinary products only)
- MSDS for constituents that are not active constituents of the product
- Certificates of analysis for constituents that are not active constituents of the product
- Consent letters for access to confidential commercial information (CCI)
- Consent for use letters for information subject to limits on use (data protection)
Where applicants choose to consolidate/compile the above-mentioned information into a single document they must:
- ensure the document is prepared as a genuine compilation containing a cover page and index (table of contents)
- name the document appropriately with the name appearing on the cover page (and being accurately reflected in the information list). An appropriate name is one that reflects what the document contains, such as ‘GMP certificates’, ‘MSDS non-active constituents’, ‘Certificates of Analysis non-active constituents’ or ‘Consent letters’.
This approach is only acceptable to the APVMA for the types of information specifically mentioned above. All other pieces of information submitted with an application are to be distinctly identified in the information list.
4.2. Authorising party
The authorising party is defined by the Agvet Code to be ‘a person who would be entitled to bring an action for breach of an obligation of confidence if the information were disclosed by someone else to the APVMA for the purposes of this Code without the person’s permission’. It is a requirement of the Agvet Code Regulations that the authorising party for each piece of information submitted to the APVMA with an application be included in the information list, as it is important to identify who can grant consent for the disclosure or use of the information, should consent be required.
The APVMA is aware that some applicants and authorising parties are concerned that the inclusion of authorising party details in the information list may identify a commercial relationship between the applicant and authorising party. Applicants should be mindful of this, and consider carefully which party is the authorising party for information developed for, and submitted with applications in their commercial arrangements.
For certain types of information such as consent letters, it may be most appropriate for the applicant to be the authorising party rather than the third-party company that is the signatory to the document. Such documents are specific to the application for which they are submitted, as they state the name of the applicant to which consent is being given and for what purpose (i.e. for the registration variation of product name only). They cannot be used for any other purpose other than to support the specific application for which they are intended. Where such information is elicited by the applicant, or on the applicant’s request, it may be most appropriate for the applicant to be the authorising party.
5. The information list should accurately reflect the information submitted
If the information contained in the information list is not complete or does not accurately reflect the actual information submitted with an application, the information list will not meet the application requirements. Deficiencies in an information list may result in delays in the evaluation of the application, and an extended assessment period prescribed by the Agvet Code Regulations may apply. This is typically one and a third of the original assessment timeframe.
6. Completing the information list
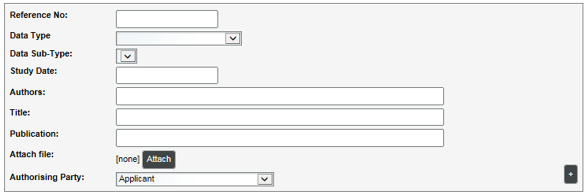
The information below provides guidance for how each field of the information list template in the application portal or Information List Editor should be completed. Complete all fields. Blank fields will prevent the information list from uploading to the APVMA record.
|
Field |
How to complete |
|---|---|
|
Reference No. |
The reference number for the information must be a unique identifier for the item of information that indicates the location of the item of information in an application. This may be the volume and page number where the item of information is located in the application or some other relevant identifier such as an index reference.
This information will not be published by the APVMA but assists the APVMA in processing the application by helping its assessors locate the item of information within a submission. |
|
Data Type |
Select the data type from the drop-down list. This will determine the available options for the Data Sub-Type. For information that is relevant to more than one data type, select the primary (main) data type only. Do not create 2 entries for one report. |
|
Data Sub-Type |
Select the data sub-type from the drop-down list. If a sub-type category is not available that accurately describes the type of information, select the closest option available.
The data type and sub-type information assist the APVMA to identify information that may be relevant to specific types of assessments. |
|
Study Date |
Enter the date that is included on the information. This will be the date on which the document was completed. If the information is published, enter the date that it was published. |
|
Authors |
Enter the name of the author(s) of the information. The author is usually the person or persons responsible for the production of the information.
It is not appropriate to enter ‘Anon’ as the author where the person or persons responsible for the production of the information is identified in or on the information or could be discovered with reasonable effort. |
|
Title |
Enter the title as it appears on the report/document. |
|
Publication |
This field only needs to be completed if the information has been published.
If the report/document is publicly available, enter the name of the publication that it was published. Please use the full journal name rather than the common abbreviation. If it was published on the Internet, then provide the Internet address (URL). |
|
Authorising Party |
Select the authorising party type from the drop-down list. Five options exist which are explained below (the term Authorising Party is defined above):
|
7. Using the information list tool in the online application form
The information list tool in the online application form allows applicants to generate the information list and upload each item of information to support an application, concurrently. It also includes a provision for the applicant to develop the necessary information list entries, and inform the APVMA that they will submit an electronic copy of the information supporting the application via post.
New information list entries can be created by clicking the + button on the bottom right-hand corner of the template.
8. Using the information list editor
The Information List tool in the online application form is the preferred method for developing an information list. However, an Information List Editor is available as an alternative way to support applicants to develop an information list where they choose to submit the information supporting their application by electronic media (CD or USB) under a separate cover, rather than uploading it through the application portal.
In order to use the Information List Editor, applicants must first download it from the APVMA website. Once downloaded and unzipped, simply double-click on the InformationListEditor.exe to start the program.
8.1. Creating an information list
To create an Information List, select ‘New Document’ from the ‘File’ menu. If you have previously begun work on a data list and wish to reopen that file, select ‘Open’ from the ‘File’ menu.
Before creating an information list it will be necessary to complete the ‘Applicant Details’. Enter the details exactly as they are written in the online application form for:
- applicant’s name
- product name or name of active constituent
- product number or active constituent number if known. If not known leave the field blank
- application number if known. If not known leave the field blank.
Do not include Registered (®), Trademark (™), Copyright (©), degrees (0) or long dash (–) symbols in the name field, which will prevent the information list from uploading.
8.2. Entering the information list entries
Once the ‘Applicant Details’ are complete, applicants can enter information into the Information List table. To begin entering information, click the ‘Add’ button on the right-hand side of the template. Follow the guidance information above to complete each field.
To add a new record, click the ‘Add’ button and repeat the data entry process for the new record. To edit a record, select an item from the Information List table by clicking on it once and then click the ‘Edit’ button on the right-hand side of the template to open the record for editing. When editing is complete, click the OK button.
Where the data package associated with an application includes similar reports or pieces of data, applicants may find it to be more efficient to duplicate an existing record and edit the copied record as required to avoid retyping the same information into multiple records. To duplicate a record, select the data item record from the Information List table by clicking on it once. Click the ‘Duplicate’ button on the right-hand side of the template. The record will duplicate and the ‘Edit’ function can then be used to edit the record as required.
To delete a record, select the item from the Information List table by clicking on it once and then click the ‘Delete’ button on the right-hand side of the template. The record will be deleted.
8.3. Saving the information list
It is recommended that users save the information list regularly whilst compiling it, and ensure that it is saved once completed. To save the information list, select ‘Save’ from the ‘File’ menu. Enter an appropriate file name and select a suitable location for the file to be saved before clicking the ‘Save’ button.
8.4. Printing
To print the information list, select ‘Save as PDF’ from the ‘File’ menu. Enter an appropriate file name, select a suitable location for the file to be saved, and click the ‘Save’ button. The pdf file will automatically open and can be printed.
8.5. Exporting to excel
Should users wish to view the completed information list in a spreadsheet format, the Information List Editor can export the information list table to Microsoft Excel. You will need to have Microsoft Excel installed on your computer for this to work.
To export the information list to Excel, click the ‘Export to Excel' button on the right-hand side of the template. Enter an appropriate file name and click the ‘Save’ button to save the file to a suitable location. The Excel spreadsheet will automatically open.
8.6. Submitting information lists generated using the information list editor
The APVMA will only accept the ‘.xml’ file of the information list. Do not submit a PDF version or a Microsoft Excel (‘.xls’) version. Submission of PDF or Excel files will prevent an application from progressing. It is an application requirement that all applications, other than applications for chemical products that are the same as a registered product or for listed products, be accompanied by an appropriate information list.
When using the Information List Editor, the ‘.xml’ file of the information list must be received by the APVMA within 7 days of the application being submitted via the online application portal. If the information list is not received within this time, the application will be refused on the basis that the application requirements were not met. There is one option through which the ‘.xml’ file may be submitted to the APVMA:
- Email:
- Attach the ‘.xml’ file to an email. Type the product name/active constituent name, and if known, the APVMA product or active number and application number in the Subject heading, and send to DataList@apvma.gov.au.
- When using the Information List Editor and submitting it to the APVMA via the above methods it is good practice to indicate in the application that the information list will be submitted by email and by whom (particularly if it is intended to be submitted by a third party).
9. Overseas data submissions
Overseas assessment packages (e.g., EU dossiers) which include all underlying data are acceptable for submission. An index or cross-reference document (e.g. an Excel spreadsheet) must be provided that clearly identifies each data item, the relevant assessment area (e.g. chemistry, environment) and the page number of the item within the dossier. Overseas assessments must be submitted in English.
The APVMA will confirm during preliminary assessment if all data items have been included in the data list, and are summarised/listed in the cross-referencing document referred to above.
10. How can I get assistance?
Should you have any questions about preparing information lists please contact APVMA enquiries by email or phone +61 2 6770 2300.
1A reference active constituent or reference chemical product is defined by the Agvet Code Regulations to be an active or product that is referred to in an application because the information that is relevant to the active constituent or chemical product is also relevant to the current application.