Table of contents
- Definition
- General requirements
- Export intervals
- Markets for consideration in export slaughter interval determination for cattle, pigs and sheep
- Data requirements and application layout
- Format for information for public consultation
- Major export food commodity groups
- Commodities generally accepted as stockfood
- MRLs in overseas countries
1. Definition
This guideline outlines the Australian Pesticides and Veterinary Medicines Authority's (APVMA) requirements for the submission of information and data relating to the impact on overseas trade of residues of agricultural and veterinary (agvet) chemicals in food commodities in support of an application for registration of an agvet chemical product. Information and data submitted in this part of the application is used in the residues evaluation and assessment of the product.
For information on all other overseas trade matters, including residues in wool and non-chemical matters, refer to other trade aspects in the special data section of the regulatory guidelines.
This section explains our requirements for the submission of information for trade evaluation and for publication and consultation on trade issues. The requirements provide for a combination of measures that maintain public consultative arrangements while also allowing more targeted notification of actions to specific primary industry sectors and government agencies at a level consistent with likely trade risks.
1.1. Reference materials
The details of documents referred to in these instructions are given under ‘References’ below. Since many of the documents are updated regularly, you should ensure that you obtain the latest editions of reference materials.
1.2. Background
The presence of residues in an export commodity above the standards set for that commodity by an importing country can adversely affect trade. Australia has experienced a number of damaging episodes that have interrupted trade following the detection of residue levels above those allowed in the importing country, particularly in livestock commodities.
Maximum residue limits (MRLs) of chemicals in food commodities are established by countries where the chemicals are approved and used in accordance with their approved uses. MRLs (which are also known as ‘tolerances’ in some countries) can therefore vary from country to country due to different use patterns and other factors. Consequently, legitimate use of a chemical in Australia, including adherence to the registered or approved use pattern, can give rise to residues that exceed the standards of importing countries while complying with Australia’s standards.
The APVMA considers potential trade issues as part of the registration process for agvet products.
1.3. The APVMA’s regulatory obligations
In considering applications for the registration of agvet products, we are obliged under the Agricultural and Veterinary Chemicals Code scheduled to the Agricultural and Veterinary Chemicals Code Act 1994 (Agvet Code) to be satisfied that the use of the product according to the registered use pattern would not unduly prejudice trade or commerce between Australia and other countries.
We consult relevant parties, including Australian Government and state and territory government authorities and grower or producer organisations, before approving an agvet product when trade implications are relevant. This consultation includes the publication of a notice of relevant information relating to the chemical product and its use in the APVMA Gazette. We may also publish separately a summary of the relevant information – for example, a Trade Advice Notice (TAN) or Public Release Summary (PRS) inviting public submissions before the registration or variation approval of an agvet product.
The publication of a notice in the Gazette and a TAN or PRS are routinely required for establishing an MRL for any compound–commodity combination where an MRL does not currently exist in Australia, or where changes to product registrations result in an increase in existing MRLs for a commodity of major trade significance.
2. General requirements
2.1. Applications requiring overseas trade or commerce information
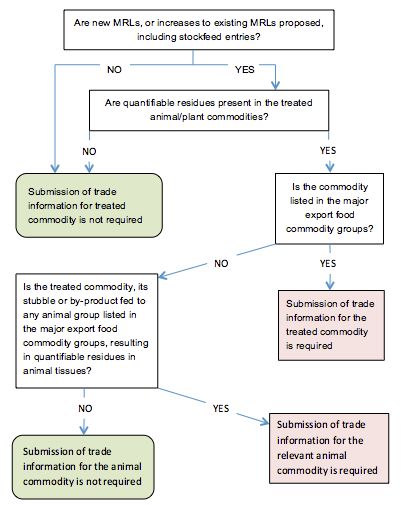
The proposed use of a new product or the extension of use of a registered product on the commodities listed in the major export food commodity groups section requires the submission of trade information for those commodities if residues are above the analytical limit of quantification. The key commodities listed in that section have been selected on the basis of both the volume of trade (expressed as a dollar value) and the potential impact that the presence of residues in a commodity would have on Australia’s total export trade.
The use of a product on commodities not listed in the major export food commodity groups section will not require trade information unless:
- a stockfood derived from treated materials has residues above the limit of quantification and produces residues in animal tissues when fed to an animal group listed in that section, or
- treated pastures and crops that can be fed to, or grazed by, stock have quantifiable residues and produce residues in animal tissues when fed to an animal group listed in that section.
The commodities generally accepted as stockfood and the major export food commodities identified may be amended from time to time. If so, these changes will follow consultation and publication of the revised information in the APVMA Gazette.
Full trade information (described below) is required when either quantifiable residues:
- are present in any treated commodity listed in the major export food commodity groups (trade information is required for each such commodity), or
- in livestock result from the feeding of treated commodities or stubbles or by-products from treated crops (trade information is required for both the animal commodity and the stockfood commodity if it is listed in the major export food commodity groups).
Figure 1 shows how to determine whether data will be required.
Figure 1: Decision tree showing when trade data should be provided with an application
2.2. Communication of trade information
The APVMA must be satisfied that the registration of an agvet product will not lead to undue prejudice to trade. We consider a range of factors, including:
- whether a potential trade risk exists (for example, due to inconsistencies between Australian and trading partners’ MRLs)
- your proposals to minimise an identified trade risk
- the capacity of affected industries to manage any such risk.
Acceptable short-term and longer-term strategies to manage identified trade risks include:
- the establishment and effective communication of export intervals
- the establishment of bilateral import tolerances
- alternative industry-specific management strategies.
We encourage you to consider making a submission to the Codex Alimentarius Commission for the establishment of an appropriate Codex MRL. You should consult with both the user industry and any other affected industries when developing the proposed strategy for communicating trade advice.
We require you to address the communication of the trade information to all relevant stakeholders in all relevant commodity production chains. Communication should occur by including appropriate export intervals or generic export statements on labels, supported by any or all of the following:
- An entry on a website database.
- Communication in a brochure.
- Communication through the company’s information phone line.
Commodity industries have developed, or are developing, mechanisms to facilitate the effective whole-of-chain communication of trade advice. Trade risk management mechanisms, such as vendor declarations, are widely used for livestock and stockfoods. We will take particular note of how your proposed communication strategy integrates with industry measures to manage trade risks when determining whether we are satisfied that a product’s registration will not cause undue prejudice to trade.
For commodity industries not included in the major export food commodities groups section, a statement on the label may be the only means of alerting the user to the possibility of trade risks through the use of the product.
2.3. Export trade advice on product labels
The APVMA and commodity industries support the free availability of information on export intervals. All methods of promoting and communicating export intervals are encouraged, including information on the label, education campaigns, website listings, published lists, point-of-sale material, quality assurance programs and vendor declarations.
We consider the inclusion of trade information on the label to be essential where chemicals are used on livestock and crop commodities listed in the commodities generally accepted as stockfeed section.
Where export intervals are proposed or required to minimise trade risks, we expect you to include the export interval value on the label, unless more appropriate industry-based strategies have been determined in consultation with the commodity industry. More information about export intervals is in the export intervals section.
We will consider alternative proposals for the communication of export intervals. Any alternative proposals must:
- integrate with strategies that minimise trade risk already implemented by users
- integrate with strategies that minimise trade risk already implemented by commodity industries
- facilitate an effective whole-of-chain communication of trade information.
Where time beyond the withholding period is not required to minimise trade risk, and hence an export interval that is different from the withholding period is not required, we expect you to include on the label a statement to that effect, such as:
- Export slaughter interval – same as the withholding period.
Where specific export intervals are not required or included on a label (for example, where affected commodities are not listed in the major export food commodity groups section), we require the inclusion of an appropriate generic statement on the label of all products used on food crops or stockfoods if residues above the limit of quantification are present at the end of the withholding period.
Suggested generic trade advice statements are as follows.
- On products used for direct application to food crops:
- Export trade advice – treated crops: Treated crop commodities destined for export may require extra time between application and harvest to be accepted in some export markets. Before you use this product, you are advised to contact [company name] and/or your industry body about any potential trade issues and their management.
- Where appropriate, the following statement should be used in conjunction with the ‘Export trade advice – treated crops’ statement, if a product’s use on food crops raises animal commodity trade issues. The advice must cover both the grower, who may also produce livestock, and any other livestock producer who may feed affected material; for example, someone to whom the grower sells treated material or someone who agists stock on the treated crop or stubble.
- Export trade advice – livestock: Consumption by livestock of any materials previously treated with this product may produce residues in the animal that might not be acceptable in some export markets. Before you use this product, you are advised to contact [company name] and/or the relevant livestock industry body about any potential trade issues and their management. You should also be prepared to inform other livestock producers who intend to use the material as stockfeed of its chemical exposure history.
These statements aim to inform the user of possible trade issues associated with their use of an agvet chemical and to provide sources of further information to identify and manage trade risks.
3. Export intervals
Export intervals are important tools in the management of undue prejudice to trade. They are advisory (non-statutory) periods proposed in conjunction with the affected grower or producer industries and the agvet chemical industry.
Export intervals assist producers, growers, processors and exporters to comply with the import standards of trading partners when they are lower than Australian MRLs, or when trading partners have not set MRLs for the particular chemical-commodity combinations. They will be either the same as, or greater than, the relevant withholding period.
Export intervals will normally be set to ensure that the exported product meets the lower of either the Codex MRL or the lowest residue standard observed by a major trading partner. For cattle, pig and sheep meat, liver and kidney, the major trading partners to be considered are listed in the markets for consideration in export slaughter interval determination for cattle, pigs and sheep section.
Export intervals must not be confused with Australian withholding periods, which relate only to Australian MRLs. A withholding period statement is a statutory statement required to appear on approved labels as part of the Australian use pattern for agvet products.
The APVMA requires adequate residues data to establish the withholding periods and export intervals.
Data required to allow the determination of an export interval should show the chemical’s depletion down to the lowest MRL or tolerance among the major trading markets for that commodity. When a trading partner has no established MRL or tolerance, the target value is the analytical limit of quantification. Consequently, we require data for the extended period required for residues to comply with the MRLs of all major markets.
You are expected to consult users and affected industries to determine export intervals that are practical and manageable for the affected industries. Four different types of export intervals can be defined, depending on the nature of the chemical product and the type of food commodity involved:
- Export slaughter interval
- Export harvest interval
- Export animal feed interval
- Export grazing interval (relating to continuous grazing)
In some cases, it will be possible to provide chemical users and affected industries with several export interval options. For example, with treated stockfoods, there may be alternatives of observing either an export animal feed interval before grazing or cutting the feed, an export grazing interval, or an export slaughter interval.
We encourage you to provide and communicate all available export interval options.
We ask you to propose an export interval in your application based on the residues data and the lowest MRL or tolerance of the major trading partners for the commodity.
3.1. Export slaughter interval
The export slaughter interval is the minimum time that should elapse:
- between the last treatment of an animal with a veterinary chemical product and the slaughter of that animal for export, or
- after the removal of grazing livestock to clean pasture or feed and slaughter, where the livestock have been grazing the crop or pasture before the expiry of the export animal feed interval.
3.2. Export harvest interval
The export harvest interval is the minimum time that should elapse between the last application of an agricultural chemical product (pesticide) to a crop and the harvesting of the commodity for export.
Where a treated commodity (such as wheat grain) may be either exported or used in Australia as a stockfood for animals destined for export, it may be necessary to determine both an export harvest interval and an export animal feed interval.
3.3. Export animal feed interval
The export animal feed interval is the minimum period that should elapse between the application of a chemical to a crop or pasture and grazing or harvesting of the crop or pasture as stockfood for animals intended to be slaughtered for export.
Where a treated commodity may be either fed to animals destined for export or exported as a commodity, it may be necessary to determine both an export animal feed interval and an export harvest interval.
3.4. Export grazing interval
The export grazing interval is the minimum period that should elapse between the application of a chemical to a crop or pasture and the slaughter of animals for export, where those animals have continuously grazed the treated crop or pasture from the time the chemical was applied.
There could be some situations in which the required export grazing interval is unsuited to normal animal, crop or pasture management. It may be necessary to determine and observe an export slaughter interval where animals are removed to untreated feed, to enable management of tissue residues for trade.
4. Markets for consideration in export slaughter interval determination for cattle, pigs and sheep
The Codex MRL standard and the standards of the markets in Table 1 for meat (fat), kidney and liver will be considered in trade assessments and in establishing export slaughter intervals for cattle, pigs and sheep.
|
Standard |
Cattle |
Pig |
Sheep |
|---|---|---|---|
|
Codex |
Yes |
Yes |
Yes |
|
China |
Yes |
||
|
European Union |
Yes |
Yes |
|
|
Japan |
Yes |
Yes |
Yes |
|
Republic of Korea |
Yes |
||
|
Russia |
Yes |
Yes |
|
|
Saudi Arabia |
Yes |
||
|
Singapore |
Yes |
||
|
Taiwan |
Yes |
||
|
United Arab Emirates |
Yes |
||
|
United States |
Yes |
Yes |
5. Data requirements and application layout
To enable the APVMA to make its decision on residues in trade and commerce matters, we need relevant information in applications for all chemicals that are used on any of the food commodity groups listed in the major export food commodity groups section. The information is required where the establishment of, or an increase in, any Australian MRL will be the subject of public consultation.
The only exceptions may be where an MRL for a commodity is reduced or where quantifiable residues do not occur. If the product is already registered overseas but has a different use pattern and/or MRLs, it is likely that we will require detailed trade information with the application for inclusion in the public consultation.
5.1. Products used on crop commodities not traded
For products used on crop commodities not listed in the major export food commodity groups section, and not used as stockfood, you do not need to submit trade data for the commodity. However, you should include a generic trade statement on your proposed label.
5.2. Products used on commodities not traded and fed to livestock
You do not need to submit trade data for commodities not listed in the major export food commodity groups section. However, trade information is required for the affected animal group(s) if the treated commodity or its stubble or other by-product is used as stockfood and contains quantifiable residues.
Details of the data requirements are shown in Table 2. Animal transfer data are also required (for further information refer to the animal transfer studies guideline). You should include generic trade statement(s) on your proposed label.
5.3. Products used on commodities traded, with quantifiable residues not expected
You do not need to submit trade residues data for products used on commodity groups listed in the major export food commodity groups section if the residues data demonstrate that there are no quantifiable residues at the expected withholding period in either the treated commodities or in any stockfood.
5.4. Products used on commodities traded, with quantifiable residues expected
Trade residues data are required where quantifiable residues occur in a commodity listed in the major export food commodity groups section, and where MRLs are either being established or are expected to be raised from their present value as a result of the direct application of the product to the commodity or animal. Table 2 lists the trade data requirements and format.
Trade information is also required for any affected animal group(s) if the treated commodity or its stubbles or other by-products:
- is used as stockfood
- produces quantifiable residues in animal tissues when fed to any animal group listed in the major export food commodity groups section.
Animal transfer data are also required.
5.5. Data requirements
You should include all pertinent information to demonstrate that, when the chemical product is used as proposed, and due account is taken of relevant residue management strategies, food commodity residues will comply with residue standards that currently apply in relevant export markets.
Each subject specified in Table 2 should be addressed in the manner indicated in the table.
|
Subject |
Information required |
Comments |
|---|---|---|
|
1. Table of contents |
A listing of the sections included in the submission and their page numbers. |
For those sections where you do not provide definitive information, you should retain the subject heading and explain why you have not provided such information (for example, ‘Not considered relevant’ or ‘No information available’). You should give a reason or justification for all statements you make. |
|
2. Summary |
A brief summary of the overall situation. Identify the food commodities concerned and the countries to which Australia exports the commodities. Specify whether any potential trade problem exists with the countries concerned. Indicate the nature of any potential problem. |
Provide an overview of the proposal. Include general information as well as a brief summary of all data or information supporting the proposal. The information should assist the APVMA (and other authorities and growers) to evaluate particular features of the product that might cause potential trade problems with the food commodities concerned. Clearly indicate the nature of any potential trade problem; for example, ‘Contravention of MRLs in Country A’, or ‘Use of the chemical prohibited in Country B’. |
|
3. Overseas registration status |
Indicate overseas registrations or impending registrations. Also, indicate where registration has been withdrawn. |
While this may appear elsewhere in the application, repeat it in this section. |
|
4. Use patterns in relevant overseas countries |
Indicate registered or approved use patterns in overseas countries where the commodity is traded. |
Provide relevant directions for use, including, as applicable, insect, disease, weed, pest, crop, animal, situation rate, dose, frequency of treatments, number of repeat treatments, withholding periods and critical comments.
Provide attachments if necessary; for example, of overseas registered labels (if in English). |
|
5. MRLs in overseas countries |
Specify the current relevant MRLs and residue definitions that apply in the overseas market countries. Alternatively, indicate any action taken, or planned to be taken, to obtain/amend MRLs (including ‘import tolerances’) in overseas countries. |
You can find advice about obtaining overseas MRLs in the markets for consideration in export slaughter interval determination for cattle, pigs and sheep section. |
|
6. Codex MRLs (CXLs) |
Indicate the current relevant CXLs and residue definitions.
Indicate any action taken, or planned to be taken, to obtain or amend CXLs. |
You could also include recommendations from either the Codex Committee on Pesticides Residues or the Codex Alimentarius Committee on Residues of Veterinary Drugs in Foods that are currently under consideration.
Indicate when you will be prepared to submit to the Codex Alimentarius Commission, if applicable. |
|
7. Label |
Include a copy of the label, including appropriate trade statements. |
Generic trade statements are not required when an export interval value is included on the label. |
|
8. Other relevant information |
Include any other information that you consider relevant.
Include details of any proposed trade risk management strategies and the associated communication strategy. |
If relevant, describe how any import tolerances affect other exporting (competitor) countries and how they may have dealt with such problems. Indicate results of any relevant trade risk consultations with authorities and/or grower or producer organisations. |
|
9. Export interval proposal (when appropriate) |
Provide an export interval proposal and an outline of the methodology and assumptions used in the estimation of the export interval. |
No comments |
|
10. Draft information for gazette notice and public consultation |
Provide trade advice information for consideration for inclusion in the APVMA Gazette notice, as the case may be. If you agree to early release, this information may also be used for consultative purposes. |
Providing this information allows us to consider your view for the finalisation of the APVMA Gazette notice.
Use attachments if necessary. The format for information is given in the format for information for public consultation section. |
|
11. Release of trade advice information to authorities and growers |
Indicate at what stage during the assessment of the application the draft trade advice information could be released for comment by authorities and growers. |
We encourage you to allow the appropriate early release of the trade advice information during the assessment process. |
Difficulties may be encountered in obtaining certain information, such as overseas MRLs. We will take such difficulties into account in considering applications. Nonetheless, we will necessarily rely on the information submitted in the application for our assessment. Information on how to find some overseas MRL information is given in the MRLs in overseas countries section.
Where the proposed use of the chemical product is expected to result in quantifiable residues in more than one food commodity, and the information to be submitted is different for each affected commodity, you should provide separate information for each commodity.
6. Format for information for public consultation
The information submitted for use in public consultation should use the following subject headings:
- Commodity/ies exported
- Country/ies where those commodities are exported
- Proposed Australian use pattern for the product
- Overseas registrations and use patterns
- MRLs of main overseas markets and Codex
- Proposed Australian MRLs
- Potential prejudice to trade
- Proposed strategies to minimise trade risk
- Conclusions
If you agree, this draft information can be released for consideration by authorities and growers during the assessment of the application to facilitate public consultations. To indicate consent, state clearly that you give permission to release the draft trade advice information. You should also indicate the stage of the assessment at which you consider release to be acceptable.
7. Major export food commodity groups
- Cattle
- Cattle dairy products
- Pigs
- Sheep
- Goats
- Poultry and eggs
- Cereal grains
- Citrus fruit
- Grapes (including dried grapes) and wine
- Oilseeds – canola seed and cottonseed (including derived oils and meals)
- Pome fruit
- Pulses – lupins, field peas, chickpeas, faba beans, navy beans, mung beans
- Stone fruit
- Sugar
- Oaten hay
8. Commodities generally accepted as stockfood
Table 3 lists commodities that are generally accepted as being fed to animals and the maximum proportion normally expected in the diet. The list is not exclusive and may be altered as livestock feeding patterns change.
|
Commodity |
Examples |
Proportion in diet for different species groups (%) |
|||
|---|---|---|---|---|---|
|
Cattle |
Sheep |
Pigs |
Poultry |
||
|
Grains |
Wheat, oats, barley, triticale, rice, maize/corn, millet, sorghum, rye |
100 |
100 |
100 |
100 |
|
Processed grain fractions (excluding grain dust) |
Pollard, bran, millrun, wheat germ, brewers grain, malt combings, biscuits, bread, hominy, semolina |
40 |
– |
40 |
20 |
|
Pulses or legumes |
Succulent or mature dried seed and immature pods of leguminous plants: peas (e.g. field pea, chickpea, cow pea, pigeon pea), beans (e.g. adzuki, faba, kudzu, mung, navy, winged), lentils, soya beans, lupins |
100 |
100 |
100 |
100 |
|
Oilseeds |
Cottonseed, sunflower seed, safflower seed, rape or canola seed, linseed, sesame seed |
30 |
30 |
30 |
30 |
|
Plant protein meals |
Oilseed meals, peanut meal, soya bean meal, copra meal, palm kernel meal |
30 |
20 |
30 |
20 |
|
Molasses or sugar |
Raw or processed sugar, molasses |
40 |
40 |
20 |
– |
|
Fruit by-products (does not include cannery wastes) |
Citrus pulp, pineapple pulp, fruit pomaces, grape marc, grape pomace |
20 |
20 |
20 |
20 |
|
Pasture |
Grass and legume pastures and mixed grass – legume pastures |
100 |
100 |
– |
– |
|
Fodder |
Hay or silage or straw of legumes, grasses and cereals; sugarcane tops; sweet corn cannery wastes; oilseed fodders and trash |
100 |
100 |
– |
– |
|
Forage |
Cereal forage, oilseed forage, legume forage, stubbles of legume, cereal, grain and oilseed crops |
100 |
100 |
– |
– |
|
Fodder vegetables |
Field turnips, kale, beets |
100 |
100 |
– |
– |
|
Tomato pomace |
10 |
– |
– |
– |
|
–: not currently considered to be a major stockfood for this species
For estimates of animal commodity MRLs, the following commodities are assumed to be fed at proportions not exceeding 5% of the animal diet on a dry-matter basis:
- Vegetables and their stubbles (excluding vegetables grown specifically for grazing or fodder).
- Waste fruit (excluding fruit by-products).
- Vegetable by-products (for example, potato peels).
- Cannery waste and by-products (excluding sweet corn cannery waste).
- Oils or fats (for example, vegetable oils, tallow).
9. MRLs in overseas countries
Assistance in ascertaining the MRLs that apply in overseas countries or Codex MRLs can be obtained from a number of sources, including the following websites: