The reconsideration process comprises legislative, administrative and scientific elements, all of which play an essential role in the final regulatory decision of whether to support the ongoing registration of an agricultural and veterinary chemical product. While a reconsideration may be represented schematically as following a linear process, in practice it is a complex iterative process, which relies on component inputs from diverse groups both within and external to the APVMA. Figure 1 illustrates the chemical review process in steps. This process includes phases that are mandatory under legislation implemented from 1 July 2014 (for example preparation of a work plan, Notice of Reconsideration); phases necessary to assess a chemical against our statutory safety, efficacy, trade and labelling criteria; and phases necessary for good project management (for example prioritisation, consultation).
Figure 1: The reconsideration process
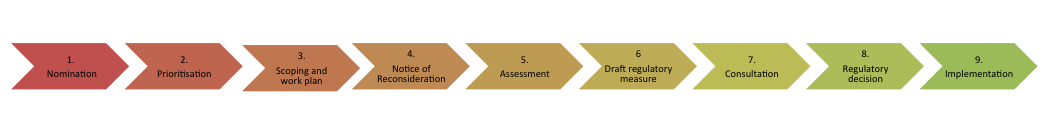
It is important to note that the above phases do not make the same proportional contribution to a reconsideration in terms of either time or complexity. The scientific assessment phase is probably the most technically complex and time-consuming, as significant amounts of data need to be evaluated in order to determine human health risks, environmental risks or both.
Critical steps prior to the formal commencement of a reconsideration (Step 4 in above diagram) are the nomination, prioritisation and planning phases.
Nomination
Anyone can nominate an approved active constituent, registered product or approved label for reconsideration. However, whether a nomination is accepted is determined entirely on a scientific basis by the APVMA in collaboration with our Commonwealth advisory agencies and/or State and Territory departments of agriculture, environment, health or primary industries.
For the majority of approved chemicals, there is already an extensive body of evidence supporting their safe and effective use and therefore a compelling scientific case is necessary to bring into question this baseline knowledge. In addition, off-label use (where a chemical is not used according to the approved product label) is not a basis for a reconsideration but would be a matter referred to the state and territory control-of-use authorities.
Following nomination of a chemical for reconsideration, the APVMA must carefully assess the new information that forms the basis for that nomination, to determine whether it contains sufficient, credible information to warrant placing the chemical under formal reconsideration.
Examples of the types of information that the APVMA would consider when considering a nomination include:
- regulatory decisions from counterpart authorities in other countries such as de-registration, restriction of use or change in use patterns. Note that in order for the APVMA to take overseas regulatory action into consideration, the products and use patterns must be relevant to Australian conditions
- adverse experience reports that have been classified as being probably related to a chemical that has been- used according to the approved label
- confirmed reports of pesticide residue violations, including trade issues such as the rejection of exported agricultural produce
- new credible scientific evidence (for example high-quality, peer-reviewed scientific literature and international scientific assessment reports by the WHO or FAO) that indicate a new or higher risk than was determined when the product was first registered
- confirmed or substantiated reports of product failure or lack of efficacy
- information submitted to the APVMA in compliance with existing statutory obligations (s. 161)
- information obtained by state and territory authorities in their administration of control-of-use functions.
A nomination must provide reasons to support the proposal for reconsideration, including any evidence of risk when used in accordance with the label instructions. If the nomination is made in response to an invitation notice issued by the APVMA, it must also conform to any criteria described in the notice.
If the APVMA’s collaborative assessment of the nomination concludes that the evidence available does not raise concerns, then the nominator would be advised that the evidence does not warrant a reconsideration. However, if the assessment shows that a reconsideration is warranted, the chemical would be accepted into the Chemical Review Program and prioritised based on its nature and the level of concern associated with it.
Prioritisation
The APVMA and our external advisory agencies use a scoring process to prioritise nominated chemicals for review, based on key criteria of concern including, human health (toxicology, public health and occupational health and safety), environment, residues and trade, target crop and animal safety, and efficacy. Further details of these criteria can be found at the page System to prioritise nominated chemicals for review.
Scoping and work plan
Historically, the APVMA has variably prepared and published scope documents for chemical reviews—more generally for the larger reviews with multicomponent inputs and quite broad and complex scopes.
From 1 July 2014, the APVMA is legislatively required to prepare work plans for all new chemical reviews (and for all existing reviews that have not been completed by 1 July 2015). These work plans will be provided to approval holders and registrants along with their section 32 notice, triggering the commencement of a review and requesting all available data to be submitted to the APVMA for evaluation. The work plan will provide predictability about the timeframe and scope for the reconsideration.
Although the Agvet Code Regulations require the work plan to be set out, the plan is not a legislative instrument. The work plan for a proposed reconsideration must include the:
- date of any notice we published inviting nominations for reconsideration
- date on which the reconsideration will begin
- proposed timeframe for the reconsideration and how the timeframe has been calculated
- matters we propose to deal with in the reconsideration
- date on which we expect to give the Notice of Reconsideration to the holder
- date on which we expect to inform any other parties of the reconsideration and invite them to make submissions, and details of who those parties are
- date on which we expect to give a notice (if any) to the holder to provide information, reports, results or samples, and a summary of the type of information, report, result or sample required by the APVMA
- date on which we expect to issue a notice (if any) proposing to vary conditions or particulars, or to suspend or cancel the approval or registration, and the anticipated recipients of the notice
- date on which we expect to make a decision in relation to the reconsideration.
The Agvet Code Regulations require that work plans be updated at least yearly, which will allow the inclusion of some of the above details that cannot be known at the time the work plan was first prepared. Events that would trigger an update of the work plan include:
- the issue of a Notice of Reconsideration (to holders or any others), or a notice requesting that the holder provide information, reports, results or samples
- a decision in relation to the reconsideration (the work plan must include details of the decision and the date of the decision)
- a variation to the label instructions (the work plan must include details of the variation)
- the issue of a permit in relation to the reconsideration (the work plan must include details of the permit)
- any suspension, cancellation or recall action in relation to the reconsideration (the work plan must include details of the action taken and the date of the action taken).
Notice of Reconsideration—the clock starts
To begin a reconsideration, the APVMA gives each approval holder and product registrant a written Notice of Reconsideration under section 32 of the Agvet Code that outlines:
- the matters we propose to deal with in the reconsideration
- the reasons for the reconsideration
- the work plan
- relevant information, including data, that the holder is required to submit to APVMA for reconsideration, and the timeframe for this to be submitted (a minimum of 28 days).The notice invites the holder to make a written submission to the APVMA, within the same period, about the matters for reconsideration.
The reconsideration starts on the first day after the expiry of the period given to the holder (to comply with the requirements of the Notice of Reconsideration). This period may be extended if there are reasonable grounds for the holder needing additional time to procure and supply the required information or data.
We may publish a notice on our website and in the APVMA Gazette, announcing the commencement of the reconsideration and giving details of the reasons for the reconsideration, scope and work plan.
Assessment
The (scientific) assessment phase of a chemical review is generally the most resource intensive and technically complex part. This phase uses an internationally accepted framework of risk analysis comprising risk assessment, risk management and risk communication.
Data submitted to the APVMA in fulfilment of the Notice of Reconsideration is scientifically assessed by specialists within the APVMA in addition to experts in the Department of the Environment. In some cases, the APVMA will contract national or international subject matter experts to provide advice on certain aspects of the risk assessment.
There is a range of data submitted to the APVMA for assessment including laboratory studies, field trials, target animal or crop studies and human studies. These studies must meet international standards of scientific quality in terms of study design and the level of reporting detail, which is necessary to allow an independent evaluation of the data.
Component assessments aim to identify the risks to human health (from dietary exposure of the general public to residues in food, and bystander exposure), workers (who use a particular product), the environment and Australian trade (through the potential exceeding of maximum residue limits) from the approved (label) use of an agricultural or veterinary chemical. Further details on these component risk assessments are described below. The conclusions of the component risk assessments are the foundation for any risk management decisions.
Chemistry assessment: The Pesticides and Veterinary Chemistry team within the APVMA undertakes an evaluation of the chemistry and manufacture of active constituents and their formulated products. This evaluation considers the manufacturing method, quality control, composition, stability and chemical characteristics of a particular active constituent or formulated product. An important aspect of this assessment is the identification of significant impurities and those of toxicological concern, and the setting of minimum standards for purity and composition.
Toxicological assessment: The APVMA undertakes an evaluation of the mammalian toxicity of an active constituent based on studies conducted in laboratory animals and observations in humans (including clinical experiments, worker exposure studies, poisoning case reports and epidemiological studies). This toxicological (or hazard) assessment comprises hazard identification, which identifies the health effects the chemical can cause at excessive levels of exposure, and hazard characterisation, which determines exposure thresholds below which no adverse health effects are likely to occur. These exposure thresholds form the basis for the setting of public health standards covering dietary exposure to pesticide and veterinary medicine residues, and the setting of occupational exposure threshold. The dietary exposure standards and occupational exposure thresholds are subsequently used by the APVMA in their residues and dietary risk assessment and in their occupational health and safety assessment. The APVMA determines whether the active constituent can be supported and recommends first aid directions, poisons scheduling and any necessary warnings for product labels.
Occupational health and safety assessment: Findings from the toxicological assessment are used by the APVMA to undertake an occupational health and safety (or worker) risk assessment. In this assessment, the estimated (by modelling) or measured (by observation or trial) level of occupational exposure resulting from the approved label use of an agricultural or veterinary chemical is compared to the occupational exposure threshold set during the toxicological assessment. Exposures below an acceptable margin from this threshold (usually 100-fold) are generally considered acceptable. This assessment also recommends safety directions, re-entry periods and restraints for all the uses supported by the assessment for inclusion on product labels.
Residues and dietary risk assessment: The APVMA’s residues team undertakes the residues and dietary risk assessment. This assessment evaluates data from laboratory studies and field trials to establish withholding periods, maximum residue limits, residue definition and restraints for all use patterns supported by the assessment. Dietary exposure to pesticide and veterinary medicine residues in food is compared to the public health standards set by the APVMA during the toxicological assessment. Dietary exposures below these health standards would indicate that the current label uses of products containing an active constituent would not lead to unsafe dietary exposure to residues.
Environmental assessment: The Department of the Environment conducts the environmental risk assessment, which considers toxicity to aquatic and terrestrial organisms (including the determination of environmental exposure limits), the fate of the chemical in the receiving environment, and the estimated or measured environmental exposures resulting from the approved label uses. The resulting risk characterisation determines whether or not the risk is acceptable and whether or not any risks might be mitigated by appropriate label advice or other action.
Efficacy assessment: If included in the scope of the review, efficacy assessments are conducted by the APVMA or independent experts external to the APVMA and rely on data generated from clinical trials or field trials on the target crop or animal pests.
Trade assessment: The APVMA’s residues team undertakes an assessment of the potential trade risks arising from all the supported uses of products. This essentially involves a comparison of the maximum residue limits set by Australia with those of our trading partners to determine their compatibility.
The APVMA and our risk assessment partners make use of all of the available data submitted to us as well as considering any relevant information that is available in the public domain. While in most cases, this information is sufficient for the APVMA to complete our assessment, there are times when additional information, testing or conducting experimental studies or trials is necessary. In this situation, the APVMA may issue a notice under section 33 of the Agvet Code requiring approval holders and product registrants to:
- provide specific information
- carry out a literature search and submit a report of the search
- conduct trials or laboratory experiments and submit the results
- provide a sample of an active constituent, a product or the product’s constituents for analysis.
At any time during the assessment stage of a reconsideration, the APVMA may take regulatory action to mitigate any risks identified in relation to the use of a chemical if we believe the action is warranted. The aim of such action is to protect human health and the environment while a final decision is being reached through the reconsideration process. The type of regulatory action taken will depend on the nature of the identified risk and the extent of harm that may occur from the continued use of the chemical. Possible actions range from amendment of instructions for use to the suspension of active constituent approvals, product registrations or label approvals. If regulatory action is taken before or during a chemical reconsideration, key stakeholders are consulted to assist with the development of a practical risk-management plan.
Proposed regulatory decision
The APVMA considers the component assessments and develops draft recommendations for the reconsideration, which summarise the results of the assessment, identified risks, risk mitigation measures, proposed review findings and proposed regulatory decision. The Preliminary Review Findings (PRF) and the assessment reports provided by the advisory agencies are released for public consultation with the proposed regulatory decision.
Consultation
The review process generally involves extensive consultation. The consultation process is designed to involve a range of stakeholders including approval holders and product registrants, users of the chemicals, peak industry bodies, interest groups, non-government organisations, the Australian Government, state and territory governments and the public. All stakeholders have an opportunity to provide information or comment on the component risk assessments, the PRF and proposed regulatory decision.
The types of information that the APVMA finds particularly useful is that which would allow any refinements to the component risk assessments underpinning the proposed regulatory decision. This information can include additional laboratory studies, monitoring data, field trials and information on current industry practices, stewardship programs and use patterns. We are also interested in understanding any practical limitations to the proposed regulatory decision and issues for product users around implementation.
Final regulatory decision
After the public consultation period has closed, the APVMA and our advisory agencies assess all the comments, information and supplementary data received and may refine the component risk assessments and risk management recommendations. We will then make the final regulatory decision, which will be one, or a combination of, the following:
Affirm the active constituent approval, product registrations and product labels.
The APVMA will affirm the approval or registration only if we are satisfied that:
- the constituent meets the safety criteria for an active constituent
- the product meets the safety criteria, the efficacy criteria and the trade criteria for a chemical product
- the label meets the labelling criteria for a label
- the constituent, product or label complies with any requirement prescribed by the regulations.
If the APVMA affirms the approval or registration, a written Notice of Affirmation is given to the holder within 14 days of the decision being made. The notice will include relevant particulars and conditions of the affirmed approval or registration. The Notice of Affirmation, including a brief statement of the reasons for the affirmation, will be published in the Gazette and on the APVMA website.
Vary the label or conditions of approval or registration
If the APVMA is not satisfied that the active constituent, product or label meets the safety, trade, efficacy an labelling criteria, the approval or registration will not be affirmed. Instead, the APVMA will determine whether the relevant particulars or conditions of the approval or registration can be varied so that these criteria can be met.
In deciding to vary the relevant particulars or conditions, the APVMA will:
- Consult with each state coordinator if the variation would affect any instructions for use, and take into account any recommendations made by the coordinators.
- Give the approval holder, registrant or any other relevant stakeholders a Notice of Proposed Decision. This notice will include a draft statement of reasons for the proposed actions, information on the basis of the proposed variations, and an invitation to provide written submissions within three months. The APVMA will take into account the submission when deciding whether to implement the variation and the specifications of any variation.
If we vary the product label before affirming the approval or registration, we will decide whether to cancel any old product labels and whether to allow a phase-out period during which the product can be used in accordance with the cancelled label.
Suspend or cancel the approval or registration
If we suspend or cancel the approval or registration, we will provide a written notice of suspension or cancellation that outlines the reasons for the suspension or cancellation. In the case of a suspension, the notice will specify the duration of the suspension and explain what actions need to be carried out by the holder for the suspension to be revoked. The APVMA will also publish a notice containing these details in the APVMA Gazette and on the APVMA website.
Implementation
The final stage of the review process involves managing the phase-out of cancelled or suspended active constituents, products, or labels and the phase-in of new product labels (if applicable). The APVMA relies on information received from stakeholders during the consultation phase in addition to information arising from targeted discussions with affected approval holders or registrants and users to determine the most practical implementation strategy.